Subproject 4
Role of the formate dehydrogenase orthologue and hydrogenases in organohalide respiration by Dehalococcoides strain CBDB1
Gary Sawers (University of Halle-Wittenberg)
Species of the genus Dehalococcoides are obligate organohalide respirers that utilize molecular hydrogen as electron donor. Strain CBDB1 not only has the capacity to synthesize 5 hydrogenases, it synthesizes a highly abundant membrane protein, which has a catalytic subunit that exhibits high similarity to 'classical' formate dehydrogenases (Fdh); however, the bacterium cannot use formate as an electron donor.
Our project has the following objectives:
- To determine the physiological function of the Fdh-like enzyme in organohalide respiration
- To determine whether the Hym-type [FeFe]-hydrogenase might form a complex with complex I in the respiratory chain
- To determine the contribution of individual hydrogenases to the hydrogen metabolism of the bacterium
- To determine whether Dehalococcoides species use an unique mechanism of electron transfer through the respiratory chain: these bacteria do not appear to synthesize quinones
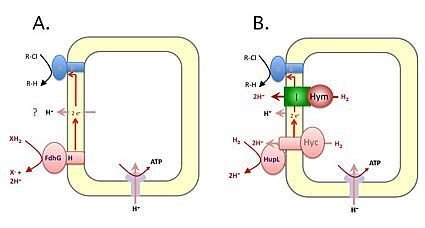 |
Respiratory electron transfer from the Fdh-like enzyme complex (A) or hydrogenases (B) to reductive dehalogenases (Rdh: represented as the blue enzyme complex). A. The route of electron transfer between the electron-donating enzyme complexes and the electron-accepting Rdh enzyme complexes, the actual electron donor to the Fdh-complex, as well as whether there is an equivalent of the 'Q-cycle' (represented by the question mark) are all unknown. B. The various Hup, Hyc and Hym hydrogenases are represented. Hym is drawn speculatively in complex with the complex I (NADH dehydrogenase) components. |
Selected References:
